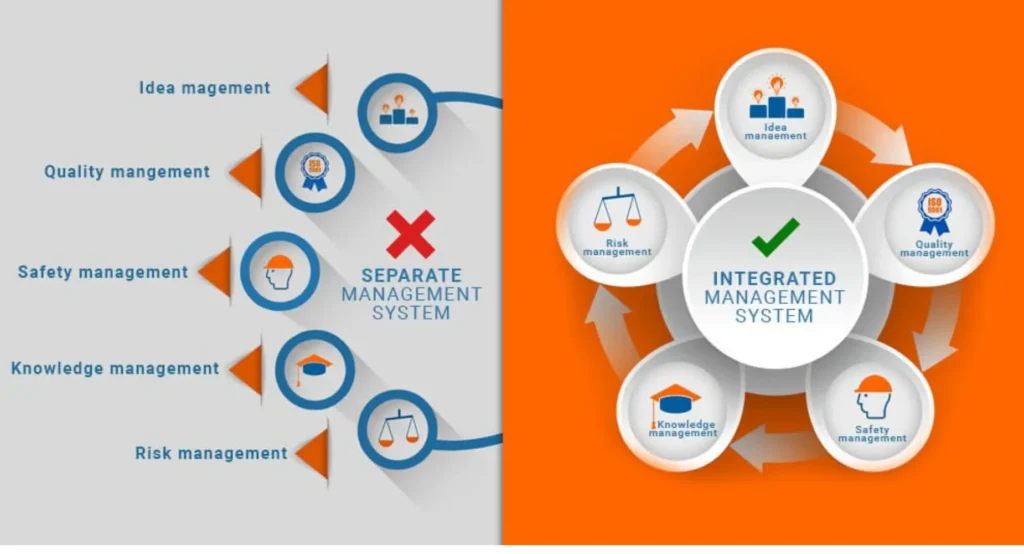
Background
A leading pharmaceutical company approached us to address inconsistencies in their management systems across multiple manufacturing units. The organization aimed to enhance compliance, improve operational efficiency, and establish a structured approach to data management. They required an Integrated Management System (IMS) that would align with multiple international standards, ensuring streamlined processes and regulatory adherence.
Challenges Faced
Before implementing the IMS, the company faced several challenges:
- Fragmented Compliance Across Units
- Each unit operated independently, following different procedures for quality, environment, occupational health & safety, and food safety compliance.
- No standardized system was in place, leading to inconsistencies in documentation and process execution.
- Manual and Disorganized Data Management
- Critical compliance data was managed manually, making retrieval difficult and prone to errors.
- Decentralized record-keeping resulted in duplication of efforts and inefficiencies.
- Regulatory Compliance Risks
- The company needed to comply with multiple international standards, but due to non-standardized implementation, compliance gaps existed.
- Preparing for audits was time-consuming and required extensive effort due to a lack of a unified system.
- Limited Transparency and Coordination
- There was minimal integration between units, causing challenges in communication and decision-making.
- Management lacked real-time visibility into compliance and operational data across units.
Our Approach
To address these challenges, we adopted a structured and phased approach:
1. Initial Company Visit & System Review
- Conducted an on-site visit to understand the company’s existing management systems and compliance structure.
- Engaged with key stakeholders, including Quality, Safety, and Compliance teams, to assess pain points.
- Reviewed documentation, processes, and data flow across all units.
2. Pre-Audit & Gap Analysis
- Performed a pre-audit to evaluate the current compliance status against international standards.
- Identified gaps in documentation, process standardization, and data management.
- Provided a comprehensive report outlining necessary improvements to align with:
- ISO 9001:2015 – Quality Management System
- ISO 14001:2015 – Environmental Management System
- ISO 45001:2018 – Occupational Health & Safety Management System
- ISO 22000:2018 – Food Safety Management System (for applicable areas)
- Good Manufacturing Practices (GMP) – As per regulatory requirements
3. IMS Implementation – Phase 1 (Pilot in One Unit)
- Developed an Integrated Management System (IMS) framework covering all relevant standards.
- Conducted training for employees to ensure smooth adoption of new processes.
- Digitized critical documentation and introduced standardized reporting mechanisms.
- Established an internal audit mechanism for continuous monitoring and improvement.
4. IMS Expansion to Additional Units – Phase 2
- After successful implementation in the pilot unit, the system was extended to two additional units.
- Standardized workflows, policies, and documentation across all units.
- Conducted additional training sessions to align all employees with the new system.
5. Centralized Data Management System – Phase 3
- Integrated all three units under a centralized data management platform.
- Enabled real-time access to compliance records, audits, and performance metrics.
- Implemented automated reporting for better decision-making and regulatory adherence.
Results & Impact
✅ Enhanced Regulatory Compliance – Successfully aligned all units with ISO 9001, ISO 14001, ISO 45001, ISO 22000, and GMP standards.
✅ Streamlined Operations – Standardized processes led to improved efficiency and reduced redundancies.
✅ Improved Audit Readiness – Easier access to compliance data reduced audit preparation time.
✅ Stronger Data Management – Centralized digital records improved traceability and reporting.
✅ Better Risk Management – Proactive compliance monitoring minimized operational risks.
Conclusion
Through the successful implementation of an Integrated Management System (IMS), we transformed the pharmaceutical company’s compliance landscape, improving efficiency and ensuring consistent adherence to regulatory requirements. By centralizing data and standardizing processes, the organization achieved greater operational control and audit preparedness.
Read this article to know about Regulatory Compliance Support for an MNC Food Brand Expanding into 11 Countries